Filing of Application for Lupus Nephritis Treatment Accepted by FDA
Aurinia’s voclosporin moves one step closer to the final decision on a potential new treatment for lupus kidney disease.
Aurinia Pharmaceuticals Inc., announced today the U.S. Food and Drug Administration (FDA) accepted the filing of the company’s new drug application (NDA) for voclosporin. The FDA now has six months to determine whether it will approve voclosporin as a new treatment for lupus-related kidney disease (lupus nephritis).
"This is encouraging news, as there are no FDA-approved treatments for lupus nephritis,” said Stevan W. Gibson, President and Chief Executive Officer of the Lupus Foundation of America. “We are proud to have supported the development of voclosporin by educating people with lupus about the importance of participating in clinical trials of this potential new drug for lupus."
Voclosporin is a type of immunosuppressive drug known as a calcineurin inhibitor (CNI). Research has shown it to be stronger, more predictable, and safer than similar medications available today. The FDA granted voclosporin priority review due to its potential to provide a significant improvement in the safety or effectiveness of treating lupus nephritis. Priority Review sets a review time of up to six months compared to a standard review time of 10 months.
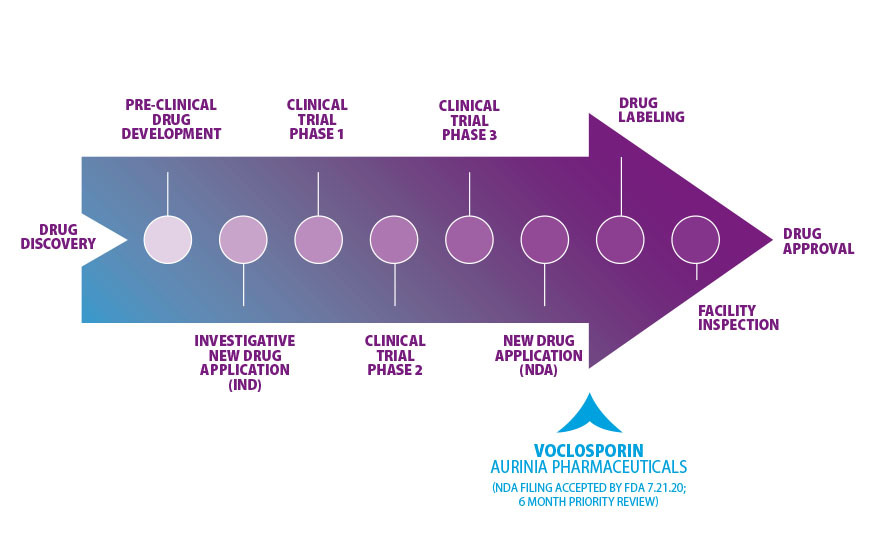
The Lupus Foundation of America will continue to monitor the progress of voclosporin through the FDA review process. We will keep you informed through Inside Lupus Research, the Foundation's news resource for developments in lupus research and treatment. For more information about today's announcement, read the news release on the Aurinia Pharmaceutical Company website.



